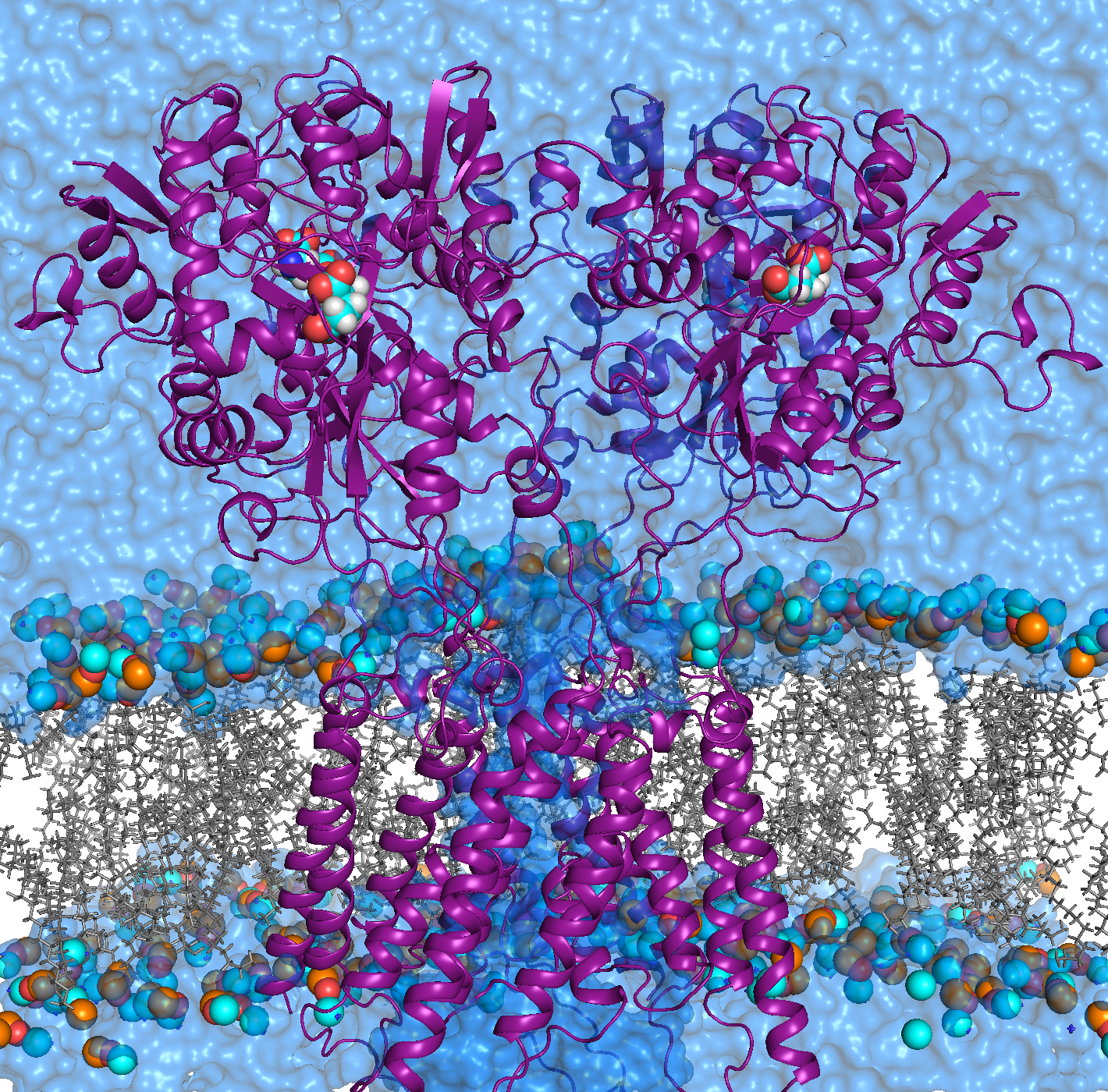
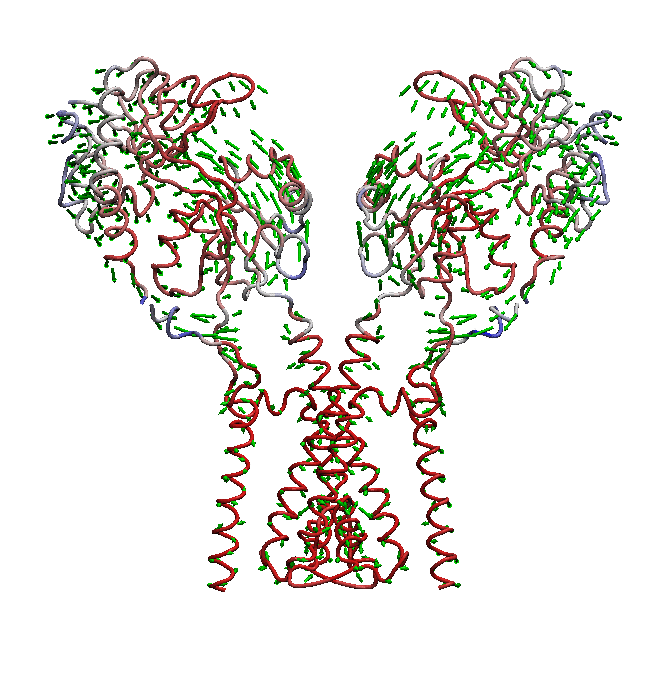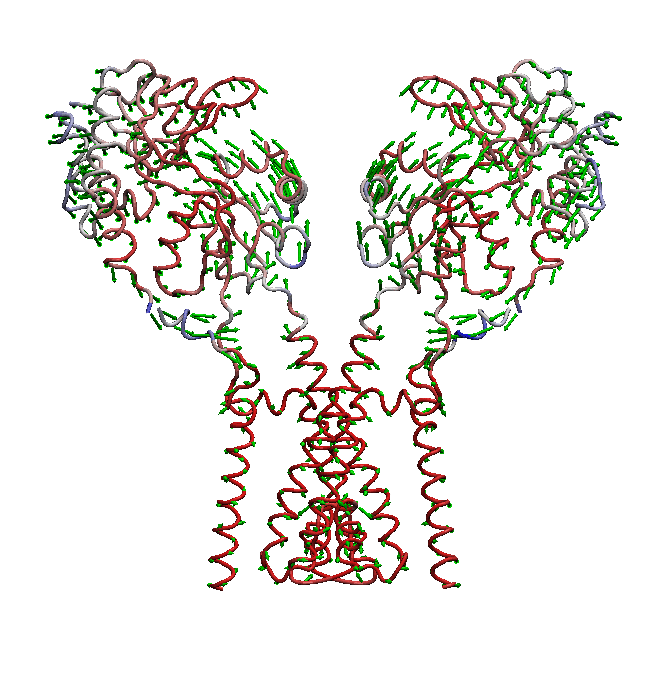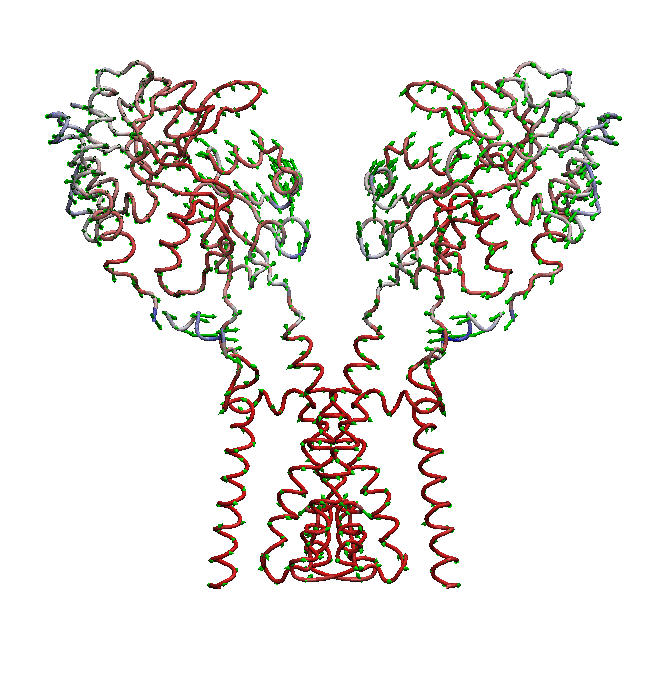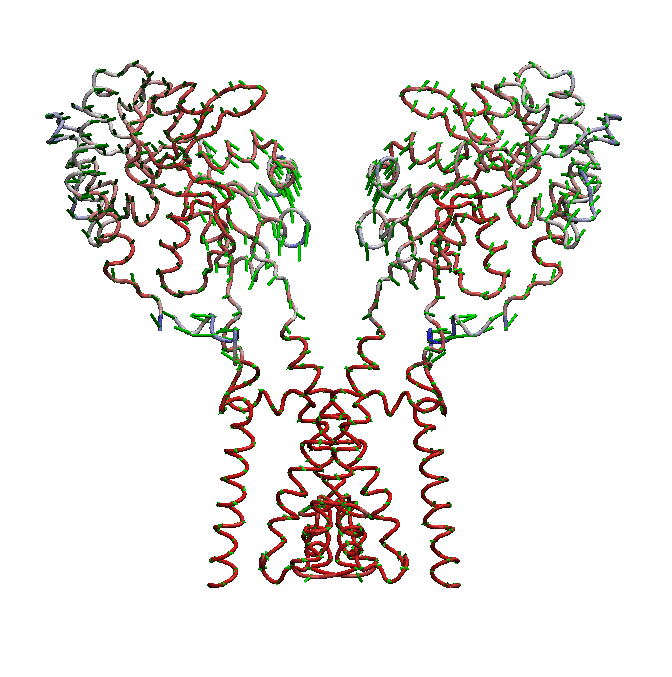
AMPA receptors (AMPARs) are members of the ionotropic glutamate receptor family that mediate fast excitatory neurotransmission in the
central nervous system and are implicated in numerous neurological diseases. Basic architecture of AMPA receptors includes a tetrameric
transmembrane domain (TMD) and a tetrameric ligand binding domain (LBD). While TMD provides the ion permeation pathway and is
responsible for the passage of substrates across the membrane, the cascade of conformational changes that leads to TMD opening of itspore
starts by the glutamate binding in the LBD.
The molecular mechanisms of how the glutamate binding in the ligand binding domain transmits to structural changes in the transmembrane
domain is not well understood. We use molecular dynamics (MD) simulations along with statistical analysis to study molecular bases
of gating (opening and closing of the receptor) in AMPARs. Specifically, our studies help to understand the correlated changes
and structural elements involved in the conformational coupling between the transmembrane domain and the ligand binding domain as the
receptor traverse from apo to open and to desensitized state.
Recent Publications:
M. V. Yelshanskaya, S. Mesbahi-Vasey, M. G. Kurnikova and A. I. Sobolevsky, “Role of the ion channel extracellular collar in AMPA receptor gating”, Scientific Reports 7: 1050 (2017)