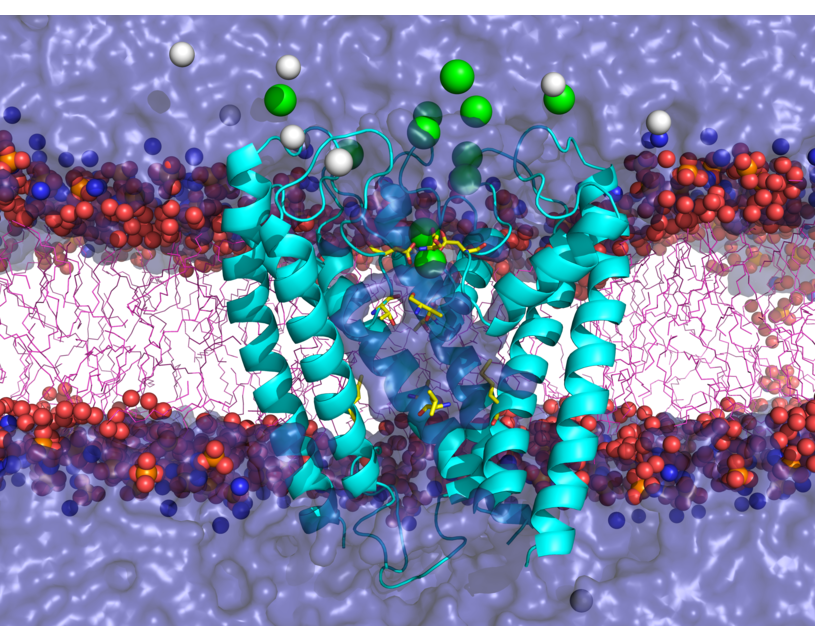
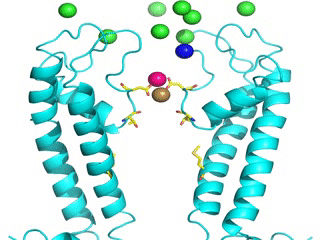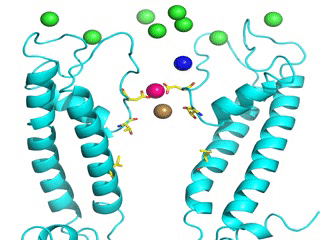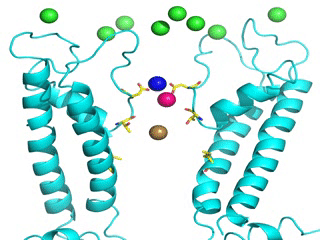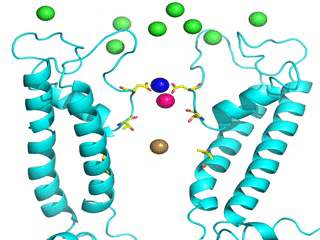
Transient receptor potential (TRP) channels function as sensors for various physical and chemical stimuli and are vitally important players in regulation of key biochemical processes in a variety of cells. TRPV5 and TRPV6 subtypes of TRP channels are the main Ca2+ entry channels in epithelia Their pores are selective for Ca2+ and permeable for a variety of monovalent and divalent cations. An exact mechanism of Ca2+ permeation and selectivity through the TRPV5/6 ion channels is unknown. Recently, a crystal structure of the TRPV6 channel, the first high-resolution structure of a TRP channel, has been solved in complex with different ions bound in the channel pore [1]. Analysis of the structure suggested that Ca2+ permeates TRPV6 channel by a knock-off mechanism. We have created a fully atomistic model of the trans-membrane (TM) domain of the TRPV6 channel in lipid and water, and performed molecular dynamics simulations of Na+ , Ca2+, Ba2+ , and Gd3+ ion permeation through the selectivity filter of this channel. As a result, our MD simulations directly demonstrate the key features of ion permeation through TRPV6 channel pore. In particular, at low calcium concentrations, we observed interplay of Na+ and Ca2+ permeation that follows the knock-off mechanism. We also compare Ca2+ permeation with permeation of Ba 2+ and channel block by Gd3+.
Publications:
Sakipov S, Sobolevsky AI, Kurnikova MG. Ion Permeation Mechanism in Epithelial Calcium Channel TRVP6 Sci Rep. 8(1):5715. (2018)
References:
1. Saotome, Kei, Singh, A. K., Yelshanskaya, M. V., Sobolevsky, A. I. (2016) Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506-511